Learning Outcomes
By the end of this lesson, students should be able to:
i. Define crude oil and its composition as a mixture of hydrocarbons with varying boiling points.
ii. Explain the concept of fractional distillation, a physical separation technique based on differences in boiling points.
iii. Describe the fractional distillation process, including the role of a fractionating column and the collection of different fractions.
iv. Identify the major fractions obtained from crude oil distillation, such as gasoline, kerosene, diesel, and fuel oil.
v. Understand the significance of refining processes in transforming crude oil into valuable petrochemical feedstocks.
Introduction
Crude oil, a naturally occurring fossil fuel, is a complex mixture of hydrocarbons with varying boiling points. Fractional distillation, a physical separation technique, plays a crucial role in transforming crude oil into a range of useful products. Understanding this process and the subsequent refining steps is essential for comprehending the production of petrochemicals, the building blocks of modern society.
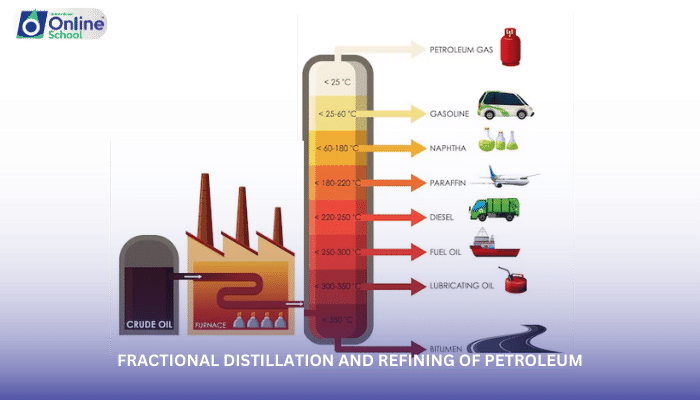
i. Fractional Distillation: Separating by Boiling Points
Fractional distillation relies on the principle that liquids with different boiling points vaporize at different temperatures. In a fractionating column, crude oil is heated to a high temperature, and as the vapor rises through the column, it encounters cooler regions. Hydrocarbons with lower boiling points vaporize first and condense at lower levels of the column, while those with higher boiling points condense at higher levels.
ii. Major Fractions: Unraveling the Crude Oil Mix
The fractional distillation process yields several major fractions, each with distinct properties and applications:
Gasoline: A volatile, low-boiling-point fraction used as fuel for gasoline-powered engines.
Kerosene: A combustible liquid used in aviation fuel, jet fuel, and heating oil.
Diesel: A heavier fraction used as fuel for diesel engines, particularly in transportation and power generation.
Fuel oil: The heaviest fraction, used as a fuel for furnaces and boilers.
iii. Refining: Beyond Separation
While fractional distillation separates crude oil into fractions, refining involves additional processes to transform these fractions into valuable petrochemical feedstocks. These processes include:
Hydrotreating: Removes sulfur and other impurities from fractions.
Reforming: Converts linear hydrocarbons into branched-chain hydrocarbons, improving gasoline quality.
Cracking: Breaks down large hydrocarbons into smaller molecules, providing feedstocks for ethylene and other petrochemicals.
Fractional distillation and refining are essential processes that transform crude oil, a complex mixture of hydrocarbons, into a range of useful products, including gasoline, kerosene, diesel, and fuel oil. Further refining processes convert these fractions into valuable petrochemical feedstocks, the building blocks for plastics, detergents, solvents, and other essential chemicals that underpin modern life. Understanding these processes is crucial for appreciating the role of petroleum as a primary source of energy and chemicals.